On the Verge of a Battery-Powered Revolution
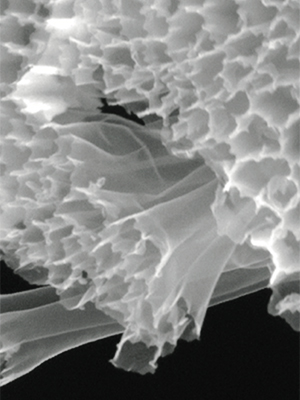
Nanostructured hollow carbon fibers for high-capacity battery electrode (Reprinted with permission from Nano Letters “Hollow Carbon Nanofiber-Encapsulated Sulfur Cathodes for High Specific Capacity Rechargeable Lithium Batteries,” coauthors Yuan Yang, Guangyuan Zheng, Judy J. Cha, Seung Sae Hong, and Yi Cui. ©2011 by American Chemical Society. Image by Guangyuan Zheng)
High-performance energy storage devices will be key to a sustainable future, allowing cell phones to go longer between recharging, increasing mileage for electric vehicles, and stabilizing the power output of solar and wind energy.
“Advanced batteries will be a game changer for addressing global challenges of energy sustainability and environmental stewardship,” says Yuan Yang, assistant professor of materials science and engineering. “Now is a really exciting time to work in batteries and energy storage.”
The world already knows the value of the lithium ion battery, the most common energy source for consumer electronics. It’s lightweight and rechargeable. But at the same time, its cycling life and energy density are still not satisfactory for various applications, including electric vehicles. It also comes with safety concerns regarding overheating, which is a problem because lithium ion batteries contain a flammable electrolyte. One promising way to overcome these disadvantages is to develop higher-capacity materials inside the battery that can safely store more energy and then deliver a higher amount of electricity.
The world already knows the value of the lithium ion battery, the most common energy source for consumer electronics. It’s lightweight and rechargeable. But at the same time, its cycling life and energy density are still not satisfactory for various applications, including electric vehicles. It also comes with safety concerns regarding overheating, which is a problem because lithium ion batteries contain a flammable electrolyte. One promising way to overcome these disadvantages is to develop higher-capacity materials inside the battery that can safely store more energy and then deliver a higher amount of electricity.
“Now a big focus of the battery community is developing high-capacity electrodes, such as metallic lithium negative electrodes,” says Yang. “That’s one major focus of my research.”
“Solar fuels are highly attractive for a sustainable energy future because they can be converted into electricity or used directly for transportation, heating, chemical processes, and more,” he explains. “Thanks to this versatility, solar fuels such as hydrogen offer an exciting opportunity for society to meet a higher percentage of its energy needs.”
Each lithium ion battery has a positive electrode and a negative electrode. The electrodes are surrounded by an organic electrolyte that contains a solution of lithium salt. Although metallic lithium negative electrode has high capacity, it tends to form thin conductive filaments, called dendrites, during the battery charging. These dendrites reduce the life of the batteries and can cause them to catch fire. In addition, lithium is so reactive that it can consume electrolytes through side reactions.
Yuan Yang is interested in designing materials and devices to address energy and environmental challenges.
(Photo by Timothy Lee Photographers)
To solve these challenges, Yang plans to observe the nanoscale processes that happen during lithium dendrite formation and develop solid electrolyte to suppress the growth of dendrites. He expects his research to advance the body of knowledge about the electrochemical reactions that happen inside the battery and to help industry design rechargeable batteries that operate at higher volumes. It could also help further understanding about how nanoscale materials interact.
“We will use some novel tools to look inside the battery—something like X-ray imaging but at a much higher resolution, such as several nanometers, which is less than 1/10,000 of a human hair,” explains Yang.
Collaboration will drive further advances in Yang’s research and be essential to enhancing energy sustainability.
“Nowadays it is hard for a single scientist to grasp all techniques necessary for investigative research, and it is also difficult for one scientist to interpret all the data,” he explains. “Collaboration is essential. It brings the expertise of different people together to tackle grand challenges of big problems, like energy sustainability.”