Simon Billinge
Nanoscience
Studying the Tiniest of Details
Just as a watchmaker is enamored with the beauty of the miniature cogs and wheels that make a timepiece work, Simon Billinge, professor of materials science and of applied physics and applied mathematics, is equally enraptured by the minuscule world of nanoparticles. By learning how these ultrafine particles between 1 and 100 nanometers in size behave within nanomaterials measured in billionths of a meter, Billinge hopes to optimize their performance and utility in biomedical, optical, and electronic applications.

Devoted to Steel
Henry Marion Howe
Former Professor
One of the first professors of metallurgy in the United States, Henry Marion Howe (1848–1922) was a professor at Columbia’s School of Mines and played a leading role in establishing the high-temperature part of the Fe-C phase diagram. His chief contribution to science and engineering was the development of the science behind the heat treatment and physical properties of iron and steel.
Howe’s Fe-C phase diagram discovery was revolutionary. The phase diagrams in materials science are essentially charts that show what chemical compounds will be stable under a variety of conditions like temperature and pressure.
As Columbia Engineering Professor Simon Billinge notes, “Phase diagrams are like maps of the conditions you need to apply in order to get the material you want.”
Howe began his academic career in his mid-30s after spending his immediate post-graduation years in industry, working domestically and abroad for iron and steel companies like Bessemer Steel Works and Taylor Iron and Steel Co. He designed and built the extensive copper smelting plants of the Orford Nickel and Copper Co. at Capleton, Canada, and at Bergen Point, NJ. He transitioned to a career in research and scholarship, first at MIT, before joining the School of Mines as professor and chair of Metallurgy in 1897. He remained at the School until his retirement in 1913.
A true pioneer, Howe was among the first to study, research, and teach metallurgy. His principal works, The Metallurgy of Steel, published in 1891, and Iron, Steel, and Other Alloys, published in 1903, were, of note, the first comprehensive books published in the field. Howe received numerous distinctions for his work and advancements in metallurgy, including induction into the National Academy of Sciences, the American Academy of Arts and Sciences, and the New York Academy of Sciences.

Professor Billinge places an object on a goniometer and bombarded it with a tiny but intense beam of X-rays that scatter as it turns. The scattered X-rays are collected on a detector and processed computationally to make an image of the nanostructure.
In order to see inside nanomaterials and learn how nanoparticles evolve, Billinge and a team of researchers at Columbia Engineering, the U.S. Department of Energy’s Brookhaven National Laboratory, the European Synchrotron Radiation Facility, and the University of Manchester combined computed tomography with X-ray atomic pair distribution functions to create a novel dual-imaging method that allows scientists to peer inside objects and view nanoparticles at work. By combining the two imaging methods, nanostructure signals can be separated from different parts of a material, giving scientists a view of how the atoms are working, without dismantling the object.
“We were really excited when this imaging method worked and immediately recognized it could have a transformative impact on the study of materials,” Billinge says. “Now we are excited to apply it to many different systems and to watch materials in action deep inside devices.”
His discovery would not have been possible if not for 19th-century Columbia professor and researcher Henry Marion Howe and his high temperature Fe-C phase diagram. Howe was America’s earliest metals researcher, and his phase diagram is the scientific basis for control of iron and steel in manufacturing.
“Phase diagrams tell you what chemical compounds will be stable under particular imposed conditions such as temperature, pressure, magnetic field, and average chemical composition,” Billinge explains. “The phase diagrams are like maps of the conditions you need to apply in order to get the material you want.”
For the experiment, Billinge and his team turned palladium metal nanoparticles into palladium oxide nanoparticles by flowing oxygen gas over them at high temperature. They then converted the nanoparticles back to palladium by flowing hydrogen gas over them. Information from phase diagrams helped determine what temperature and gas composition to use to make this work.
Although Billinge and his team were fairly certain their theory regarding the dual-imaging process would work, they were astonished by the actual images.
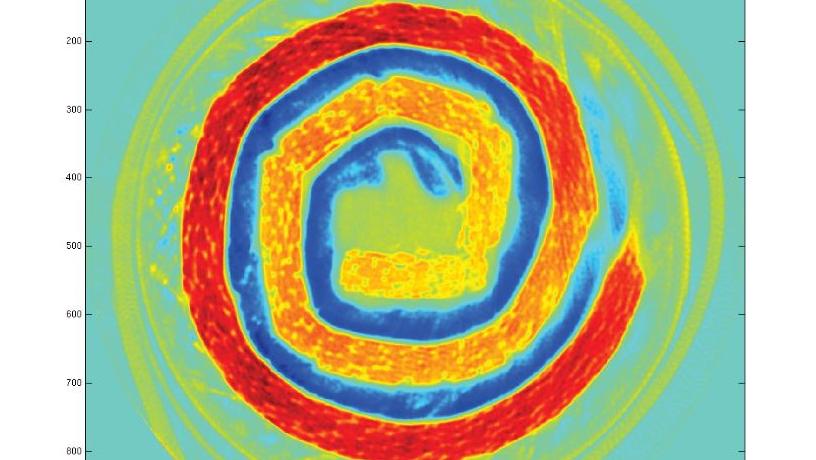
A nanostructure image of the inside of a rechargeable NiMH AAA battery. Each of the colorful pixels contains a complete mathematical map of the nanostructure.
“It was totally magical when the first images came in and we were able to get a complete nanostructure determination in each pixel of the image,” Billinge says. “The surprise was seeing how precisely we could chart out the structural nature of tiny nanoparticles in different locations deep within the object and following how they changed under different chemical conditions.”
Billinge and his associates were equally surprised at the animation of the nanoparticles. “It was as if they had personalities and were like people,” he says. “The little ones ventured deep into the middle of the object (which was a nanoporous catalyst support made of alumina, an oxide of aluminum) and resisted being oxidized by the oxygen gas we began flowing, as if they were hiding. The big ones stuck around the edge of the object in a very thin layer, couldn’t hide, and got oxidized.”
That view into the catalytic activity helped further define how to prepare high-performing catalysts and leverages Billinge’s work in structural disorder and the development of methods for analyzing nanostructure.
“Other scientists are also working hard on this area, called atomic pair distribution function analysis,” Billinge explains. “This is becoming much more widely used for nanostructure studies and is one of the most powerful tools in the bottom-up nanotechnologist’s toolkit.”
Billinge and his team are at the forefront of developing the tools to push the study of nanoparticles and materials further, and are excited about the potential for more discoveries.
“It is not hard to stay motivated because new discoveries in nanoscience come one after the other when you develop tools at the forefront of nanostructure analysis,” he says. “This regularly gives us new insights into how real materials do their work, for example, while absorbing light or transporting electricity, heat, or ions. We rarely cease to be surprised as we uncover the mechanisms materials use to carry out these tasks.”