Latha Venkataraman
Nanoscience Getting a Charge Out of Nanotechnology

Ever since the dawn of the electronic age, science has been on a quest for miniaturization, making transistors smaller in order to integrate many into a circuit and further miniaturizing to build microprocessors. The potential for further miniaturization is predicated on physics: The tiniest transistors must still be large enough to control the on or off flow of electrons. The smallest transistors that can be envisioned consist of just a few atoms or a small molecule. The potential for making such small electronic circuits has captivated Latha Venkataraman, associate professor of applied physics.
“By developing circuits with components that consist of a single molecule (a collection of a few atoms), I am working at a size regime that is close to the fundamental limit,” she says. “There’s something exciting about exploring how things work at such an extreme and largely uncharted scale.”
The underlying focus of Venkataraman’s research is to fabricate single-molecule circuits, a molecule attached to two electrodes, with varied functionality where the circuit structure is defined with atomic precision. That’s the holy grail of molecular electronics and a goal Venkataraman had been pursuing even when popular science relegated the idea to theory.
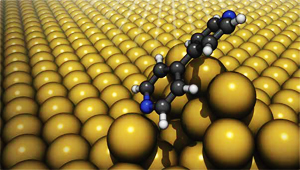
Illustration of a molecule on gold surface bonded through chemical and van der Waals interactions. The forces required to break these bonds were first measured in Venkataraman’s lab.
“When I started at Columbia, the field of molecular electronics was plagued with uncertainty. No one could imagine that a single-molecule circuit could actually function reliably and reproducibly,” she explains. Through collaboration with chemistry groups at Columbia, a method was devised to create workable single-molecule devices.
“This was a major breakthrough, because we could finally probe the properties of such circuits and relate these to the chemical and physical natures of the molecules,” she says.
Now, Venkataraman is focused on learning how these circuits work and how to optimize their function as active device components. Her findings will also enhance the understanding of charge transport in molecular systems and across metal-organic interfaces, with impact on the fields of organic electronics, photovoltaics, catalysis, and even biological processes (such as photosynthesis and respiration).
“We are now measuring how electronic conduction and single bond-breaking forces in these devices relate not only to the molecular structure but also to the metal contacts and linking bonds. Our experiments provide a deeper understanding of the fundamental physics of electron transport, while laying the groundwork for technological advances at the nanometer scale,” she says.
Improving the understanding of how molecules transport charge can help in development of next-generation devices, and Venkataraman is on the verge of technological breakthrough. A team in her lab recently substituted layered materials for a gold electrode in a molecular circuit.
“We’ve always used gold metal electrodes to contact molecules in our circuits, but when we switched to using multilayered graphene flakes as one electrode, these devices ended up functioning as diodes, which are the basic building blocks for a transistor,” she explains. That research was published inProceedings of the National Academy of Sciences this summer.
The next step for Venkataraman is to figure out how to make more interesting circuit components such as switches or optically active elements and build these into circuits. In this process she is also exploring new ways to control the interface between molecules and electrodes.
“We are working on creating molecular devices with varied functionalities that are controlled through chemical design,” she says. To understand the interplay of physics, chemistry, and engineering at the nanometer scale, Venkataraman works with a range of scientists and encourages the graduate students in her lab to get comfortable with collaboration.
“Collaboration is really what makes my research possible and fun,” she says. “Most of my graduate students and I are trained as physicists. We do not know much chemistry, and all the work that we do requires understanding molecular structures to relate them to the electronic functions that we measure. Without our spending hours talking with our chemistry collaborators, none of this would be possible.”
Engineering Icon Irving Langmuir Alumnus

A renowned chemist whose cutting-edge research incorporated engineering and physics, Irving Langmuir was a giant in his field. It all began at Columbia Engineering, where he studied metallurgical engineering at the turn of the twentieth century. He graduated in 1903, having developed a passion for understanding atomic and molecular properties, especially in relation to vacuum phenomena. Twenty-nine years later, he would receive the Nobel Prize.
After attaining his PhD in Germany for research on the behavior of dissolved gases in a new kind of electric lamp, Langmuir briefly taught at the Stevens Institute of Technology before joining the General Electric research laboratory, where he became associate director and remained for 40 years. At first his research centered on developing gas-filled incandescent lamps and improving vacuum tubes, but he delved into surface chemistry upon discovering that hydrogen formed a layer one atom thick on the surface of bulbs with tungsten and platinum filaments. A 1917 paper on the chemistry of oil films forming molecular monolayers, and his subsequent general theory of adsorbed films, led to his 1932 Nobel Prize for advances in surface chemistry.
Ever restless in his research, Langmuir developed the atomic hydrogen welding process and made important contributions to understanding valence and atomic structure. He was among the first to work with plasmas and coined the term. He also became interested in atmospheric chemistry and, after working to improve various military technologies during World War II, researched the possibilities of cloud seeding.
Langmuir served as president of the American Chemical Society, whose journal of surface chemistry today bears his name. He remained devoted to the Engineering School, supporting the campaign that funded construction of Mudd Hall and the Engineering Terrace, and his research lives on in countless technologies that remain in common use.