Clean Energy Requires Clean Storage

Recent Chemical Engineering Ph.D. graduate Anna Dorfi and Esposito discussing electrocatalysis research in the Solar Fuels Engineering Lab.
In their essence, fossil fuels are primordial batteries, the ancient remains of plants that captured the Sun’s energy millennia ago through photosynthesis and stored it in the form of chemical bonds. In the U.S., 81% of the energy we use comes from these non-renewable sources in no small part because of that fact— and because designing a more efficient platform for energy storage is no small feat.
“Storage is essential for a sustainable energy future,” says Daniel Esposito, associate professor of chemical engineering. “Renewable sources like solar and wind are inherently intermittent. Fuels are a great way to store excess energy for times when the Sun isn’t shining, and the wind isn’t blowing.”
Devices known as electrolyzers use electricity to produce chemical fuels through electrochemical reactions, and they can generate emission-free fuels if the power is sourced from renewables. Esposito leads the Solar Fuels Engineering Laboratory, which explores novel electrolyzer designs capable of turning water and carbon dioxide into fuels like hydrogen and more energy-dense organic molecules.
Electrolyzers rely on efficient catalysts to drive the chemical reaction without generating excess heat. Esposito’s team is developing composite catalyst materials that employ nanoscale silicon dioxide overlayers—i.e., ultra-thin glass—to improve catalyst durability and better control the distribution of chemical products produced through electrochemical reactions.
The researchers characterize these materials with analytical tools that allow them to establish design rules for how electrochemical reactions behave at the buried interface between the ultrathin overlayer and metallic catalysts.
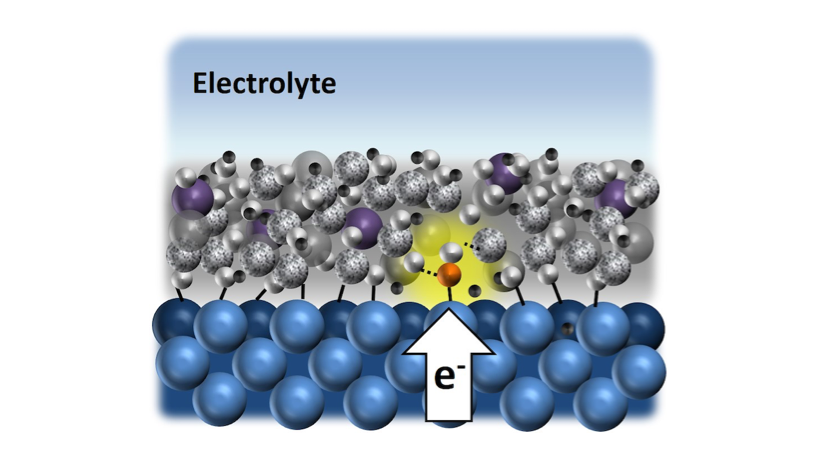
Schematic sideview of a reactant molecule located at the buried interface between a catalytic metal layer (blue atoms) and a permeable nano-membrane (grey atoms) used to control reaction pathways for renewable fuels production.
Esposito carries out this work as a core faculty member of the Columbia Electrochemical Energy Center (CEEC). CEEC colleague Jingguang Chen, Thayer Lindsley Professor of Chemical Engineering, with a joint appointment as a senior chemist at Brookhaven National Laboratory (BNL), works to advance fuel cell science as well. He investigates the fundamental properties of bimetallic and metal carbide catalysts for fuel cells and electrolyzers, with a focus on developing high-performance catalyst materials made from earth-abundant metals. His goal is to minimize the use of the expensive and scarce precious metals that traditionally provided the best catalysts—a crucial step for scalability.
Using BNL’s National Synchrotron Light Source II (NSLS-II), Chen’s group carries out spectroscopy and diffraction measurements to derive key insights into the composition and atomic structure of catalytic materials.